
As Dr. Matilsky discussed in his video lecture, atomic spectra occur due to the fact that orbital radii of electrons, and hence their energies, are quantized at specific levels determined by the atomic number (number of protons) and ionization state (number of electrons) in any given element. When looking at astrophysical objects we either see an absorption or emission spectrum. With absorption spectra we see essentially continuum emission with certain wavelengths of light missing and spectrographs usually render this as a black line.
 (from http://solarsystem.nasa.gov/deepimpact/science/spectroscopy.cfm)
(from http://solarsystem.nasa.gov/deepimpact/science/spectroscopy.cfm)
An emission spectrum on the other hand, shows little or no continuum emission, and only displays light at specific wavelengths.
 (from http://solarsystem.nasa.gov/deepimpact/science/spectroscopy.cfm)
(from http://solarsystem.nasa.gov/deepimpact/science/spectroscopy.cfm)
Whether an object will present an absorption or emission spectrum depends greatly on the geometry of the continuum source with respect to the observer on earth. Absorption spectra generally form when a continuum source, such as the central regions of a star, is directly in our line of sight, but behind our object of interest (which in this example), is the outer atmosphere of a star. Therefore we receive most of the light from the continuum source, except for those wavelengths that can promote electrons in the outer atmosphere to higher energy levels, thus removing these photons from the game.
For emission spectra, the source of the continuum is oblique to the line of sight between the observer and the object. Therefore the continuum source heats the object, and the electrons inside the atoms emit photons to move into lower energy states, which is always preferred by nature. We see examples of this in the so-called emission nebulae, which are regions of rarified gas that are heated by stars off to one side of the nebula.
Hydrogen-like atoms are those atoms with only one electron remaining, regardless of the number of protons in the nucleus. An example would be singly ionized Helium, which is the lightest hydrogen-like atom, besides hydrogen.
Niels Bohr proposed a model of the atom that explained with startling accuracy, the appearance of the spectrum of hydrogen. In this model, energy levels, En, of hydrogen-like atoms can be determined as,
$$E_n\left[eV\right]\simeq\frac{Z^2\left(-13.6\,{\rm{eV}}\right)}{n^2},\:\:\:\left(1\right)$$
where Z is the number of protons in the atom, and n is the principle quantum number (just an integer!) that represents the quantized energy levels of the orbiting electrons. Also note, that the unit of energy is eV, or electron-volts. These units are related to electric potentials measured in Volts, like your wall-socket is 120V (if you are in the USA).
The energy of an emitted/absorbed photon is set by the difference in energy levels for a jump from one energy level with a given principle quantum number nlower, to another level with principle quantum number nupper. The energies are negative because our convention is to set the zero point of energy at infinity. An electron loses energy when it comes closer to the nucleus, (just as you lose potential energy when you roll down a mountain), and so its energy in the vicinity of the nucleus is negative. The reason it loses energy is because there is an attractive force between the negatievly charged electron, and the positively charged nucleus.
Example: Balmer Lines
In hydrogen, the Balmer Series is a special name given to transitions from larger n to the n=2 state, or vice versa. For an example let us calculate the energy and wavelength emitted when the electron in a hydrogen atom goes from n=3 to n=2 energy state.
1. First note that Z=1 since there is only 1 proton in hydrogen. We can use equation (1) to get the energies at the two different levels as,
$$E_2=\frac{1^2\left(-13.6\rm{eV}\right)}{2^2}={-3.4eV,}\:\:\rm{and}$$ $$E_3=\frac{1^2\left(-13.6\rm{eV}\right)}{3^2}={-1.51eV.}\:\:\:\left(2\right)$$
2. The energy of the photon is the difference of these two states,
$$E_{3\rightarrow{2}}=E_3-E_2=1.89\rm{eV}.\:\:\:\left(3\right)$$
The wavelength can be determined by the relationship,
$$E_{\gamma}=h\nu_{\gamma}=\frac{hc}{\lambda_{\gamma}},\:\:\:\left(4\right)$$
where h is Planck's constant = 4.1×10-15 eV•Seconds, and c is the speed of light (300,000 km/s). Rearranging we find that the wavelength λ is,
$$\lambda_{3\rightarrow{2}}=\frac{hc}{E_{3\rightarrow{2}}}=656.5\;\rm{nm}.\:\:\:\left(5\right)$$
This technique will work with any hydrogen like atom. Just make sure you put Z in properly when you use equation (1). To make things more simple we can combine equations (1) and (4), and generalize the observed wavelength of a photon that results from a transitions between two different quantum "n" levels as,
$$\lambda_{n_{_{upper}}\rightarrow{n_{_{lower}}}}=-\frac{1}{0.011\,Z^2\left(\frac{1}{n_{_{upper}}^2}-\frac{1}{n_{_{lower}}^2}\right)}\;\rm{nm}.\:\:\:\left(6\right)$$
Note that the answer to equation (6) is always a positive value for the photon's wavelength. This is not only the wavelenth for a photon emitted from transitioning from a higher to lower energy level, but is also the wavelength of a photon need to stimulate an electronic transition from a lower state to a higher one.
Any object with a Temperature greater than Absolute Zero, will radiate energy away in the form of light. A Blackbody Spectrum is what would result if you had a perfectly black box that had a set temperature, and you observed the intensity of light at various photon energies. This is important because we can often treat astrophysical objects like stars to be near-perfect blackbody emitters. So we can model the continuum emission upon which we see the absorption spectra. What this means is that besides being able to observe the chemical make-up of a distant star, we can also determine its temperature near the surface.
Now for some hands-on experience!
The University of Colorado's education department has developed a great tool, the Blackbody Spectrum Simulator. Click on the download button, and opening the downloaded file should open the applet in your browser as seen below.
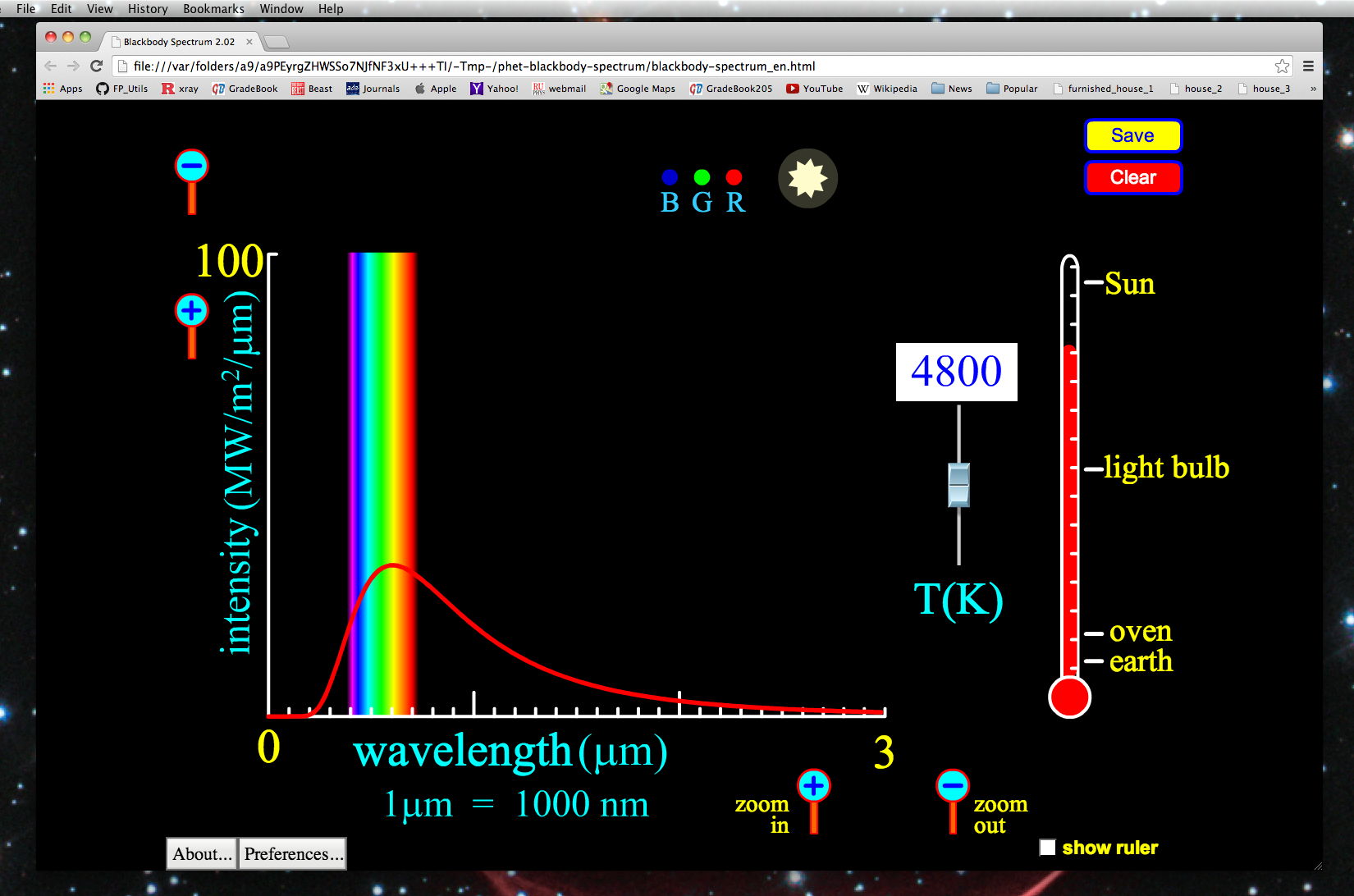
Let us discuss what we are seeing here in the plot. The x-axis represents the wavelength of light being emitted and the y-axis represents the intensity, or strength of emission of the blackbody radiation at a given wavelength. Note the units! Blackbody radiation is almost always given as energy per unit surface area of the object. There is a rainbow shown on the x-axis at the actual wavelengths of visible light.
You can change the temperature with the slide-bar just left of the thermometer on the right, or enter a temperature (in Kelvin) in the white box above the aforementioned slide-bar.
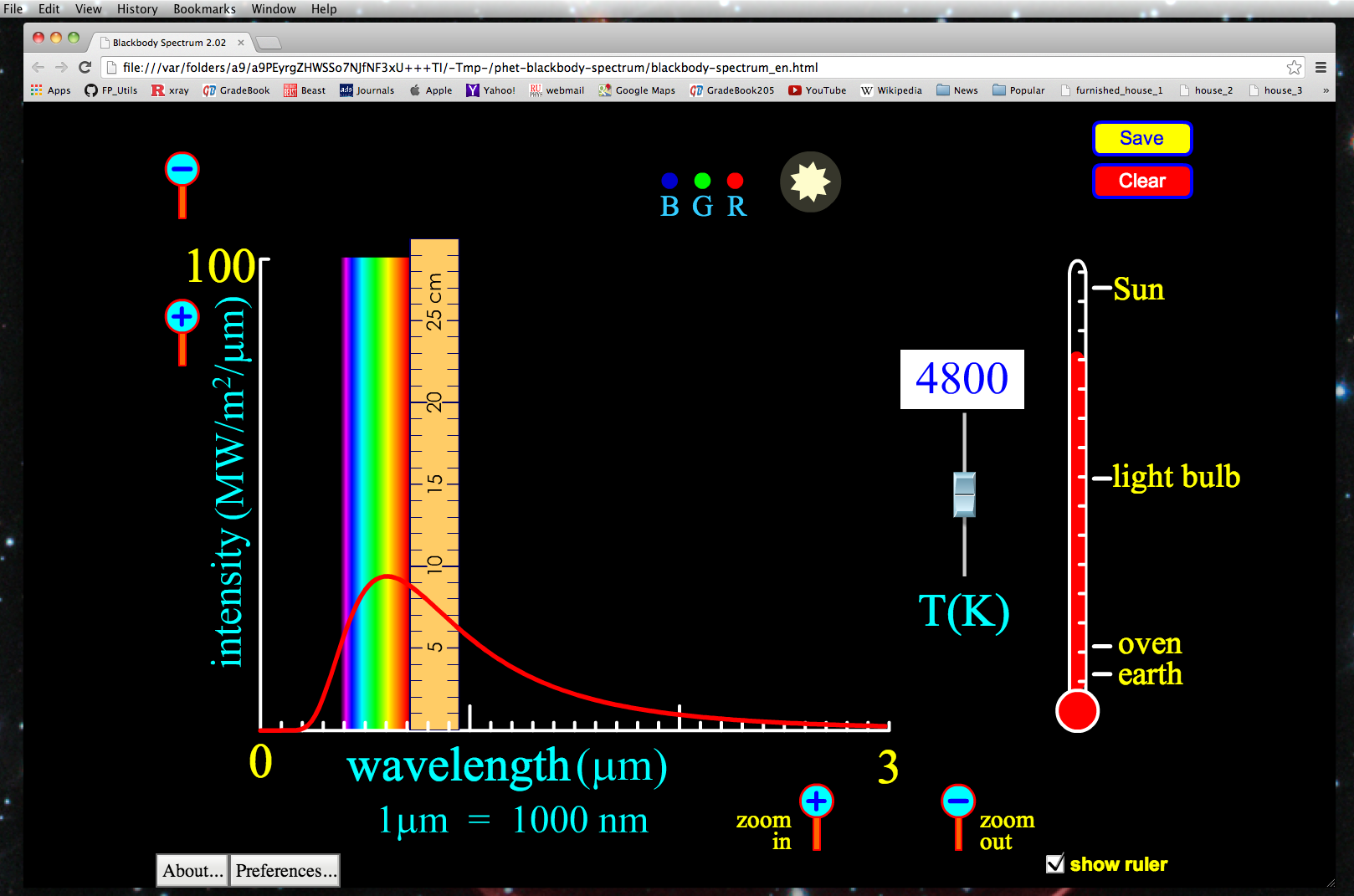
Now notice we can click the "show ruler" box at the bottom-right of the simulator. This brings up a ruler as shown in the figure below, which allows us to measure the height of the intensity at a given wavelength of light. Note that if you use the zoom in or zoom out buttons the scale of the ruler DOES NOT change. Therefore if you wish to examine relative strength of a certain wavelength of light being radiated, you cannot zoom-in or zoom out while doing this.
Exercise:
1. Click the zoom buttons until your x-axis runs from a minimum of zero, to a maximum of 1.5 micrometers. Next display the ruler if it is not already, and move the left-edge of it to be on the 0.7 micrometer hash-mark, while placing the bottom of the ruler flush with the x-axis.
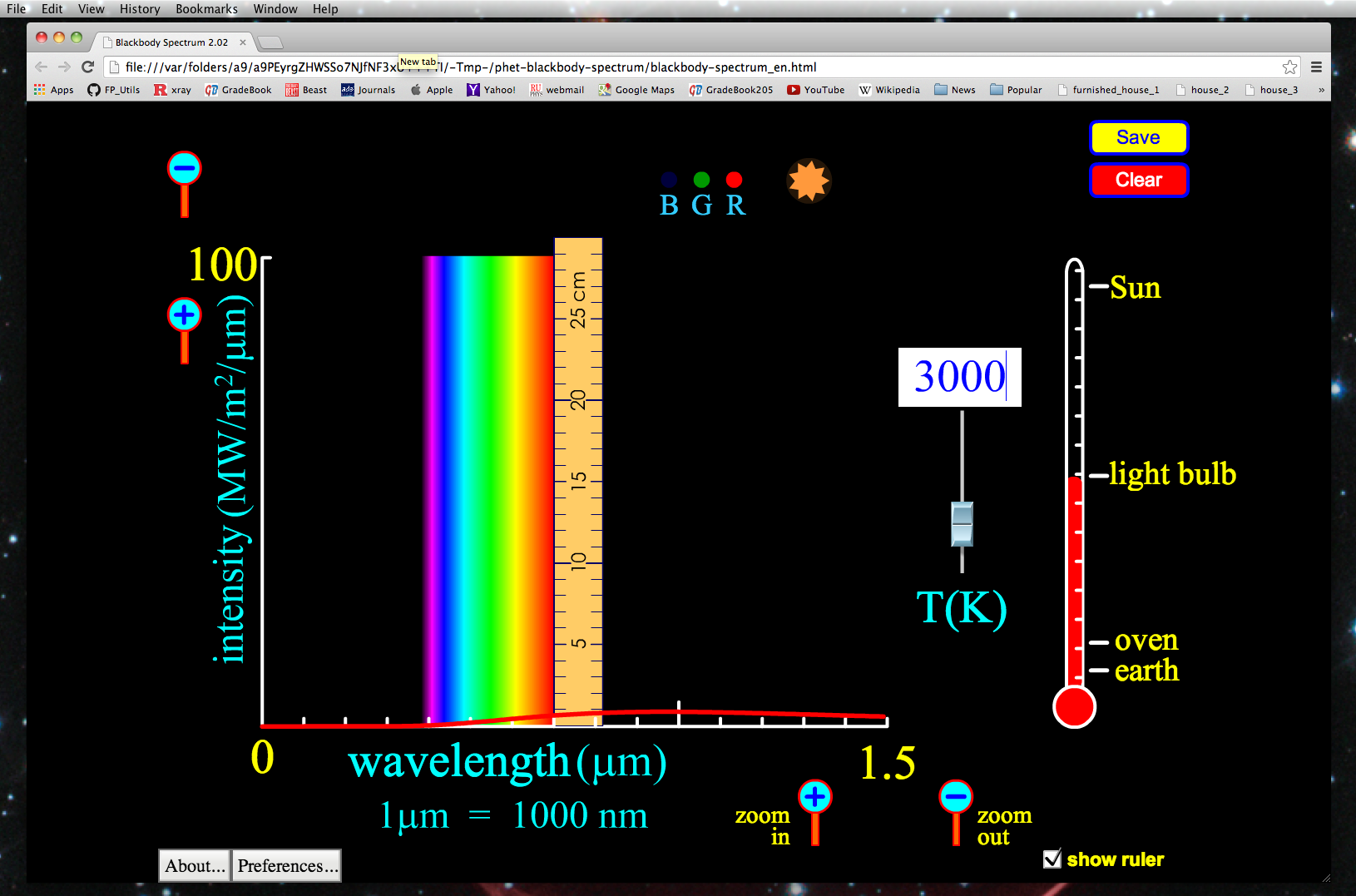
2. Enter a temperature of 3,000 K. Note the wavelength where the intensity is at its highest and note the "height" of the emission at the 0.7 micrometer wavelength.
(You should find something like the maximum intensity occurs at a wavelength of about 1 micrometer, and the height of the emission is about 0.7 cm. )
3. Now repeat step 2 for temperatures of 4,500 K and 6,000 K.
Results: As we go through this we see that as the Temperature of the blackbody increases, the maximum intensity of radiated light takes on smaller wavelengths (greater energy). We also see that at any given wavelength the intensity of radiation emitted goes up as the temperature of the blackbody increases.