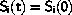
There four forms of the second law in model systems, one of these forms is the particular form observed in reality.
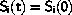
In this case the entropy is constant, there is no asymmetry between past and future and there is no thermodynamic equilibrium.
 for all t>t'
for all t>t'This form has an asymmetry between past and future, but does not guarantee that the entropy will converge.
 for all t>t'
for all t>t' 
 for all t>t'
for all t>t' 
This form has an asymmetry between past and future and the system evolves towards an equilibrium state, which is independent of the initial state.
SL-IV is the form of the second law observed in thermodynamics.
Much of the following discussion evolves around the investigation of which modification to Hamiltonian dynamics is required to change the second law for the system from SL-I to SL-IV.
Author Per Stoltze stoltze@fysik.dtu.dk