
In the follwing we will consider the mechanism

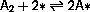
As an adsorption site, *, is either free or occupied by
A2* or A*, the coverage probabilities,
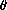 ,
must
add up to unity:
,
must
add up to unity:
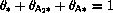
In the simplest case the reaction rates are proportional to the coverages.
Using
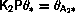

At equilibrium the net adsorption rate is by definition zero.
If we compare the rate equation to the conventional equilibrium
equation
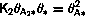
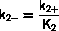
Usually both k2+ and k2- have Arrhenius form
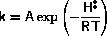
A is known as the preexponential factor while
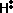 is known as the activation energy
is known as the activation energy
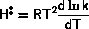
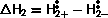
As the adsorption energy,  is negative, high pressures and low temperatures
generally favour the formation of adsorbed molecules.
is negative, high pressures and low temperatures
generally favour the formation of adsorbed molecules.
However, in the gas-phase the A2 molecule has three translational and two rotational degrees of freedom. In the adsorbed state these degrees of freedom are transformed into 6 vibrational degrees of freedom around the equilibrium position at the surface. This usually results in a negative adsorption entropy. At a given pressure the equilibrium between gas and adsorbate will shift towards desorption when the temperature is increased.