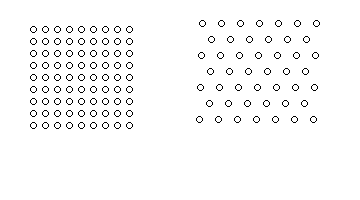Introduction
Atoms and molecules in a gas can adsorb on solid surfaces with
a wide range of adsorption energies
[1].
Depending on the energy of adsorption,
we distinguish between different types of adsorption:
- Physisorption is weak and is caused by van der Waals interactions
between the molecule and the surface.
- Chemisorption is caused by the formation of a chemical bond
between the molecule and the surface. The binding is much more specific,
the energy of adsorption range from small to very large. In many
cases the molecule dissociates during the adsorption process.
At intermediate temperatures
the adsorbed molecules usually form patterns on the surface
Two of the simplest patterns are the quadratic (left) and the
hexagonal (right) grid of adsorption sites.
These patterns are typically found on fcc(100) and fcc(111) surfaces.
Much more complicated patterns are possible.
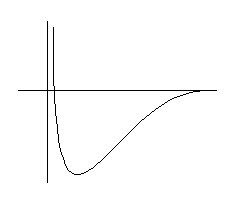
An illustration of the adsorption energy for an atom
outside a solid surface.
The abscissa is the reaction coordinate and may,
loosely, be interpreted as the height over the surface.
Actually, the whole diagram is an oversimplification. The potential
energy surface is multidimensional and depends on the height, the
position and orientation of the molecule.
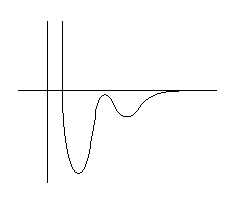
The potential energy for a diatomic molecule, A2,
outside a surface.
The shallow well is due to the adsorption in a molecular state, A2*.
The deeper well is due to the adsorption as atoms 2A*.
Start
Next
Author
Per Stoltze
stoltze@fysik.dtu.dk