X-RAY ANALYSIS OF A SOLID
Experiment Developed with National Science Foundation Funding.
Learning Experiences
- - Use of the Bragg Equation
- - Modern Chemical Analysis
- - Fingerprinting a Solid
- - Computer Graphics
Pre-Lab Question
1. List all safety precautions that must be observed in the use of x-rays.
X-Ray Diffraction Experiment
INTRODUCTION
X-rays are electromagnetic radiation of wavelength about 1 Å (10-10 m),
which is about the same size as an atom. They occur in that portion of
the electromagnetic spectrum between gamma-rays and the ultraviolet.
The discovery of X-rays in 1895 enabled scientists to probe crystalline
structure at the atomic level. X-ray diffraction has been in use in two
main areas, for the fingerprint characterization of crystalline
materials and the determination of their structure. Each crystalline
solid has its unique characteristic X-ray powder pattern which may be
used as a "fingerprint" for its identification. Once the material has
been identified, X-ray crystallography may be used to determine its
structure, i.e. how the atoms pack together in the crystalline state
and what the interatomic distance and angle are etc. X-ray diffraction
is one of the most important characterization tools used in solid state
chemistry amd materials science.
We can determine the size and the shape of the unit cell for any compound most easily using the diffraction of x-rays.
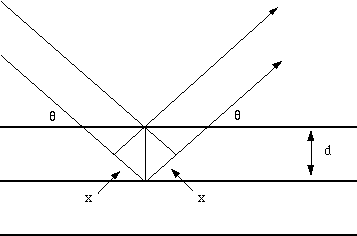
Fig. 1 Reflection of x-rays from two planes of atoms in a solid.
The path difference between two waves:
2 x wavelength= 2dsin(theta)
For constructive interference between these waves, the path difference must be an integral number of wavelengths:
n x wavelength= 2x
This leads to the Bragg equation:
n x wavelength = 2dsin(theta)
Figure 2 shows the x-ray diffraction pattern from a single crystal of a
layered clay. Strong intensities can be seen for a number of values of
n; from each of these lines we can calculate the value of d, the
interplanar spacing between the atoms in the crystal.
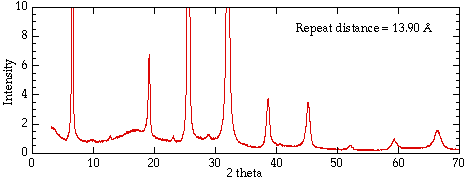
Fig. 2 X-ray diffraction pattern from a layered structure vermiculite clay.
EXAMPLE 1 Unit Cell Size from Diffraction Data
The diffraction pattern of copper metal was measured with x-ray
radiation of wavelength of 1.315Å. The first order Bragg diffraction
peak was found at an angle 2theta of 50.5 degrees. Calculate the
spacing between the diffracting planes in the copper metal.
The Bragg equation is
n x wavelength = 2dsin(theta)
We can rearrange this equation for the unknown spacing d:
d = n x wavelength/2sin(theta).
theta is 25.25 degrees, n =1, and wavelength = 1.315Å, and therefore
d= 1 x 1.315/(2 x 0.4266) = 1.541 ÅIn
this lab you will measure the x-ray powder diffraction pattern from a
single crystal. Your TA will give you the sample to be measured and
show you how to set up the Miniflex x-ray diffractometer.
You should measure all the values of 2theta from the chart, and
after converting them into d values calculate the repeat distance in
your unit cell. In your lab note book list all the 2theta values with
their corresponding values of n and d. Then calculate the mean and
median values of the unit cell.
INSTRUMENTATION
The X-ray diffraction experiment requires an X-ray source, the sample
under investigation and a detector to pick up the diffracted X-rays.
Fig 3 is a schematic diagram of a powder X-ray diffractometer.
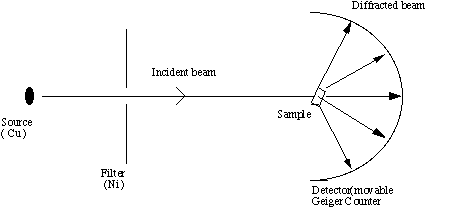
Fig. 3. Schematic of an X-ray powder diffractometer
The X-ray radiation most commonly used is that emitted by copper,
whose characteristic wavelength for the K radiation is =1.5418Å. When
the incident beam strikes a powder sample, diffraction occurs in every
possible orientation of 2theta. The diffracted beam may be detected by
using a moveable detector such as a Geiger counter, which is connected
to a chart recorder. In normal use, the counter is set to scan over a
range of 2theta values at a constant angular velocity. Routinely, a
2theta range of 5 to 70 degrees is sufficient to cover the most useful
part of the powder pattern. The scanning speed of the counter is
usually 2theta of 2degrees min-1 and therefore, about 30 minutes are
needed to obtain a trace.
PROCEDURE
**Do not open the chamber while the alarm red light is on
**Never touch the detector (may result in signal off).
- Obtain a sample from your instructor, place it onto the
double-side tape which is then placed on an aluminum sample holder; if
you are preparing a powder sample, use a spatula to spread the powder
onto the double-side tape.
- Read the instructions for the Miniflex X-ray diffractometer,
which are on the wall above the instrument. Your instructor will
explain the operation.
- Set the instrument at optimum setting as follows
time constant 2
range ?
chart speed: Low
- Slide in the sample holder and adjust the beginning 2theta at 70 degree (It scans from high degrees to low degrees)
- Switch on the start knob and chart recorder (slow) simultaneously, run your sample on slow chart speed.
- Once scan gets down to 3 degree of 2theta , stop (switch start knob to off) and chart. TURN OFF X-ray.
- Locate all peaks on the chart and corresponding 2theta values
and write their values into the data chart below. Perform the necessary
calculations in the table and calculate the repeat distance in your
unit cell.
Data Table of X-ray Diffraction Peaks
| 2theta | theta | sin(theta) | n | d=n x wavelength/sin(theta) | lattice spacing
= n x d
|
|---|
|
|
| 1 |
|
|
|---|
|
|
| 2 |
|
|
|---|
|
|
| 3 |
|
|
|---|
|
|
| 4 |
|
|
|---|
|
|
| 5 |
|
|
|---|
|
|
| 6 |
|
|
|---|
|
|
| 7 |
|
|
|---|
|
|
| 8 |
|
|
|---|
Wavelength = 1.5418 Å for Cu Ka
For more information please contact Stan Whittingham: stanwhit@binghamton.edu
Copyright © 1989-1997 M. Stanley Whittingham