|

DEPARTMENT OF PHYSICS AND
ASTRONOMY
LINKS
|

Basics: Conversion Reaction Materials for Energy Storage
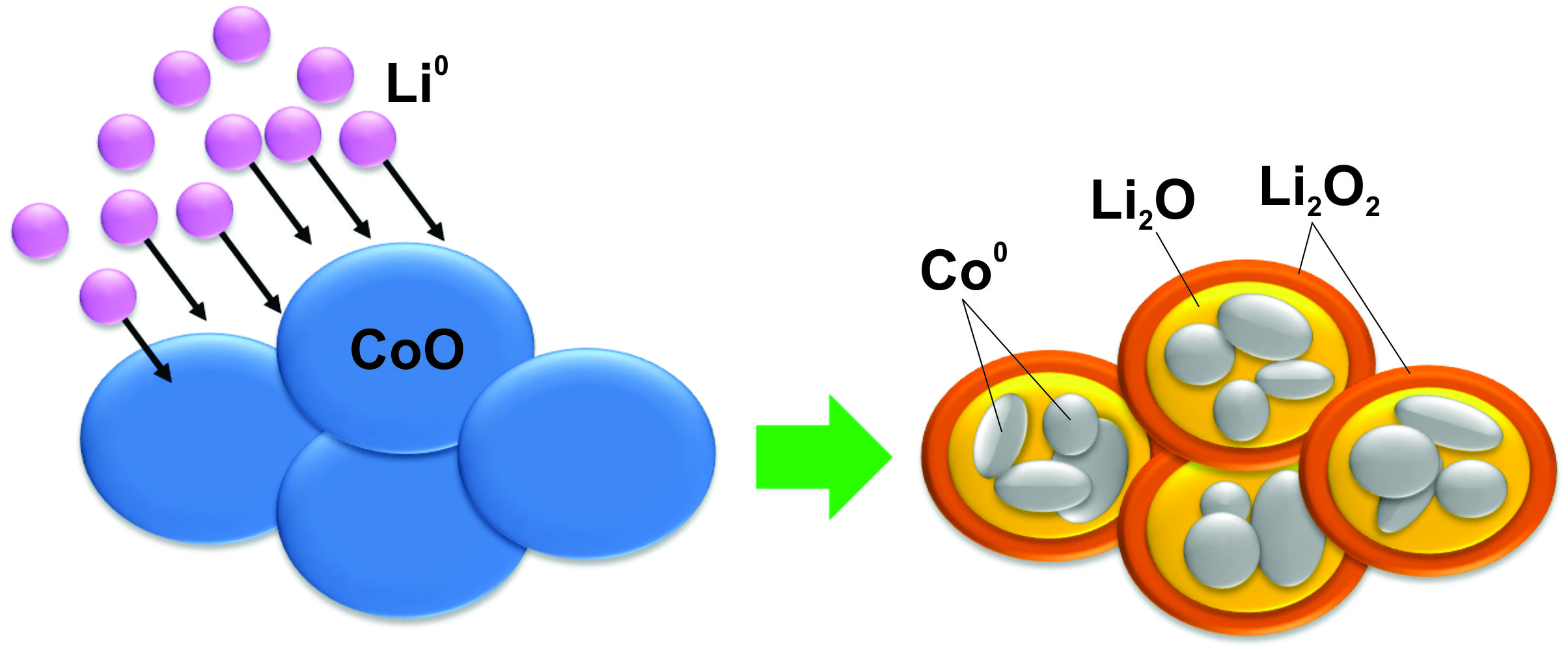 Conversion reaction compounds are a new class of materials which exhibit
high charge storage density in Li-ion battery applications. Upon exposure to
Li, these compounds are reduced from a high oxidation state to their metallic
state, thereby storing two or three electrons per formula unit. This represents
a 2- or 3-fold increase in charge storage capacity over conventional intercalation
batteries.
Conversion reaction compounds are a new class of materials which exhibit
high charge storage density in Li-ion battery applications. Upon exposure to
Li, these compounds are reduced from a high oxidation state to their metallic
state, thereby storing two or three electrons per formula unit. This represents
a 2- or 3-fold increase in charge storage capacity over conventional intercalation
batteries.
Nanophase transition metal fluorides and oxides such as FeF2
and CoO have exhibited good performance in electrochemical studies, but the
atomistics and phase evolution of the conversion reaction are not well understood.
In order to characterize these materials without contamination from atmosphere or
electrolytes, we have grown thin films of FeF2 and CoO in ultra-high
vacuum and exposed them to atomic Li to induce a conversion reaction. The
electronic structure and phase of the films before and after lithiation have been
investigated with ultraviolet photoemission spectroscopy
(UPS), inverse photoemission spectroscopy
(IPS), and x-ray photoemission spectroscopy
(XPS).
Using these techniques, we have observed the reduction of the conversion compounds
as a function of Li exposure. We have also used transmission electron microscopy
(TEM) to show that both FeF2 and CoO react with Li to form metal
nanoparticles embedded in a Li-rich matrix. Additionally, angle resolved XPS
(ARXPS) of epitaxially grown CoO and FeF2, has been used to show that
the conversion reaction front proceeds uniformly through the films from the
surface, in some cases leaving unreacted regions behind.
|
Recent Highlights: Temperature Dependence of the Li-CoO Reaction
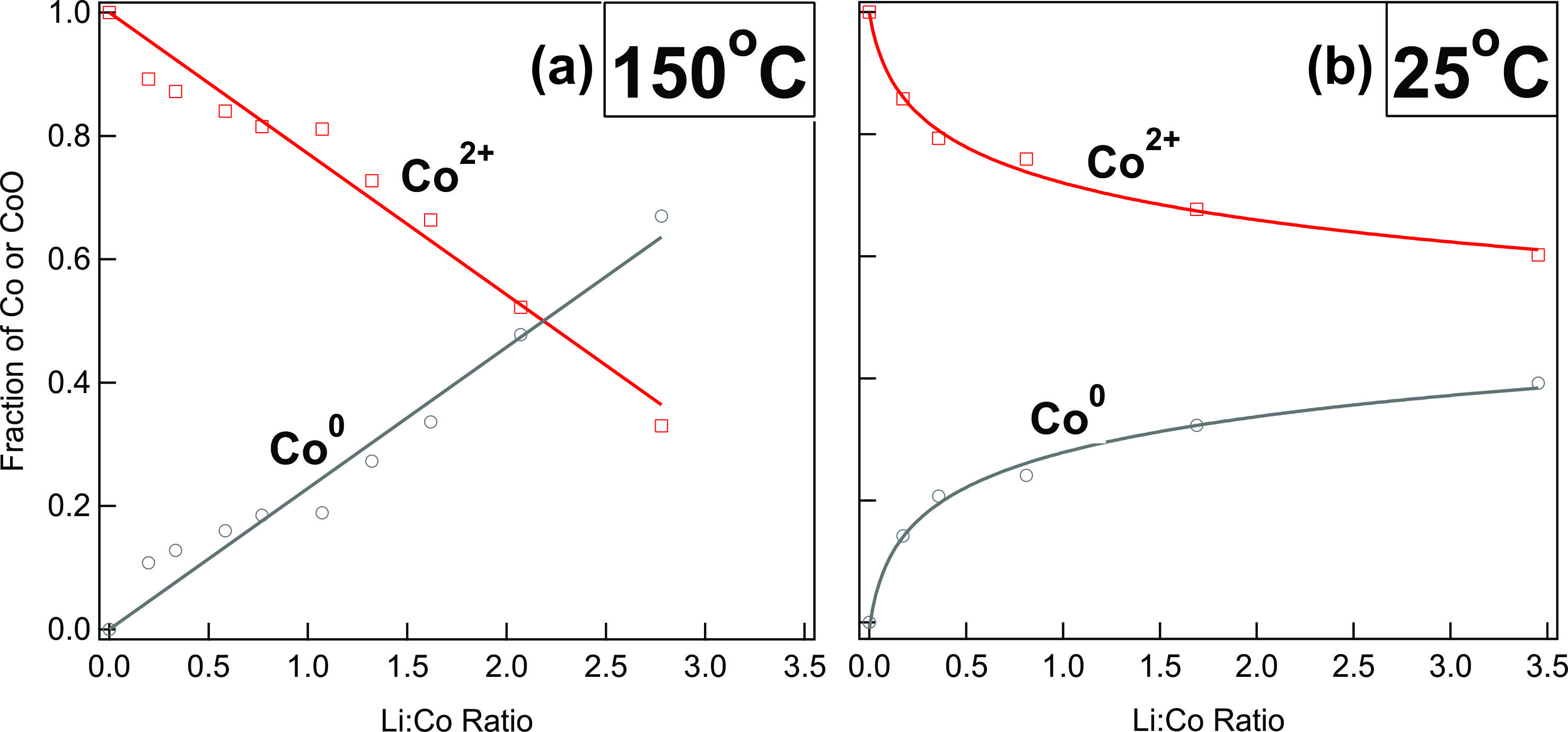
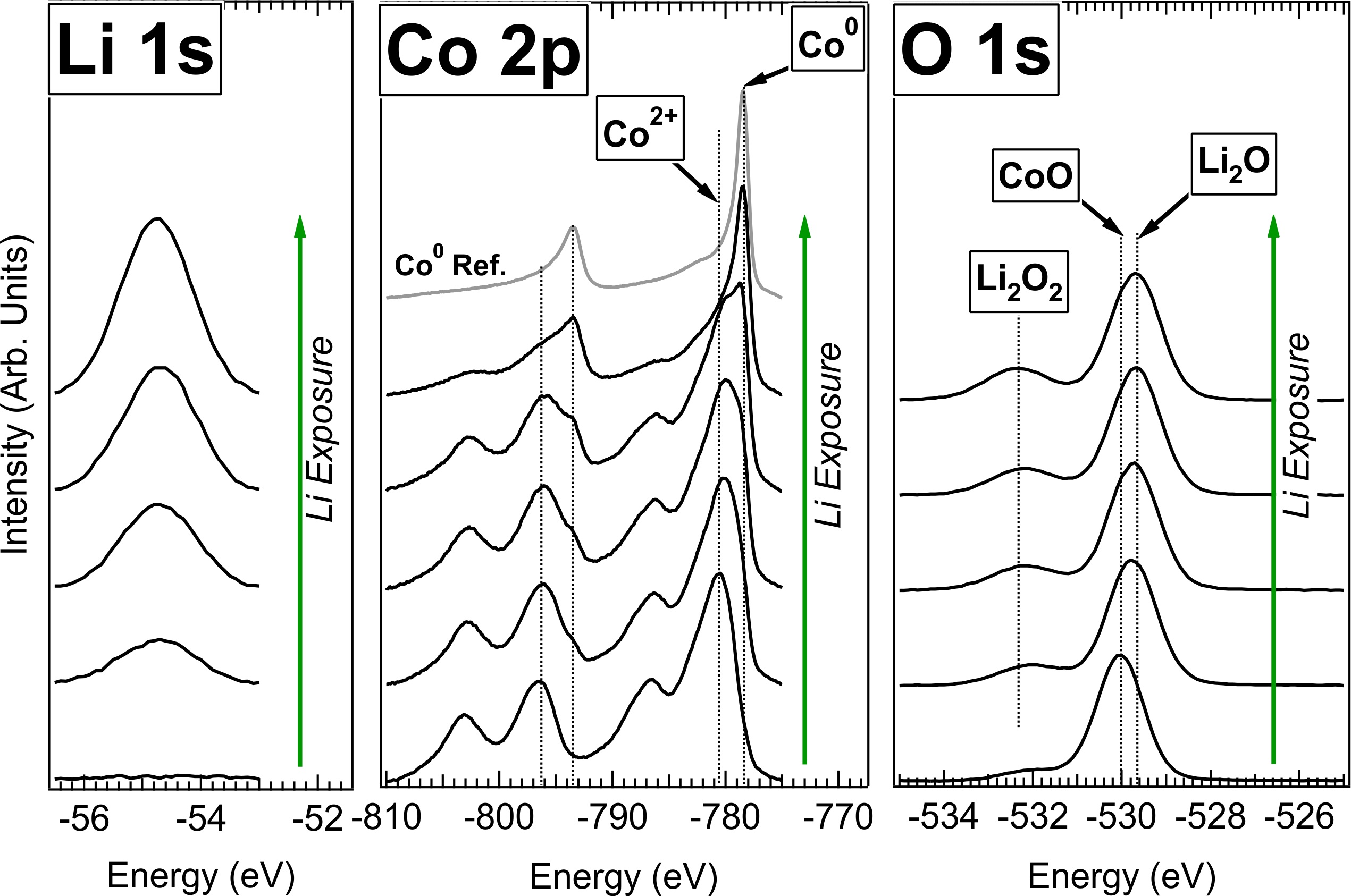 Polycrystalline CoO thin films were grown and exposed to atomic Li in vacuum. XPS of
the film before and after a series of Li exposures shows a gradual increase in
Li content concomitant with the formation of metallic Co and Li2O,
as expected from electrochemical studies. However, an additional peak in the O-1s
spectrum suggests the formation of an additional Li-oxide species due to the parasitic
reaction of Li2O with residual O2 or H2O gas in the vacuum
chamber. This compound is believed to be Li2O2, which is known to
inhibit Li diffusion at room temperature in Li-air batteries. Consequently, when the film
is lithiated at high temperature (150oC), the amount of CoO conversion is
proportional to the amount of Li deposited, whereas lithiation at room temperature
(25oC) results in a diminished degree of conversion. These results suggest that
any conversion battery whose products include Li2O is likely suffer from kinetic
limitations at room temperature.
Polycrystalline CoO thin films were grown and exposed to atomic Li in vacuum. XPS of
the film before and after a series of Li exposures shows a gradual increase in
Li content concomitant with the formation of metallic Co and Li2O,
as expected from electrochemical studies. However, an additional peak in the O-1s
spectrum suggests the formation of an additional Li-oxide species due to the parasitic
reaction of Li2O with residual O2 or H2O gas in the vacuum
chamber. This compound is believed to be Li2O2, which is known to
inhibit Li diffusion at room temperature in Li-air batteries. Consequently, when the film
is lithiated at high temperature (150oC), the amount of CoO conversion is
proportional to the amount of Li deposited, whereas lithiation at room temperature
(25oC) results in a diminished degree of conversion. These results suggest that
any conversion battery whose products include Li2O is likely suffer from kinetic
limitations at room temperature.
|
Recent Publications
(back to top)
-
Conversion Reaction of CoO Polycrystalline Thin Films Exposed to Atomic Lithium
R. Thorpe, S. Rangan, M. Sina, F. Cosandey, and R.A. Bartynski, J. Phys. Chem C 117, 14518 (2013)
-
Conversion reaction of FeF2 thin films upon exposure to atomic lithium
S. Rangan, R. Thorpe, R.A. Bartynski, M. Sina, F. Cosandey, O. Celik, and D.D.T. Mastrogiovanni, Journal of Physical Chemistry C 116, 10498 (2012)
-
Formation, dynamics, and implication of solid electrolyte interphase in high voltage reversible conversion fluoride nanocomposites
A.J. Gmitter, F. Badway, S. Rangan, R.A. Bartynski, A. Halajko, N. Pereira, and G.G. Amatucci, Journal of Materials Chemistry 20, 4149 (2010)
-
Electrochemical Performance of Acid-Treated Nanostructured LiMn1.5Ni0.5O4-d Spinel at Elevated Temperature
N.M. Hagh, F. Cosandey, S. Rangan, R.A. Bartynski, and G.G. Amatucci, Journal of the Electrochemical Society 157, A305 (2010)
-
Fabrication, physical and electrochemical investigation of microporous carbon polyiodide nanocomposites
P. Barpanda, Y. Li, F. Cosandey, S. Rangan, R.A. Bartynski, and G.G. Amatucci, Journal of the Electrochemical Society 156, A873 (2009)
|
|