

LINKS
|

Basics: Dyes Sensitized Solar Cells; Energy Alignment Concept
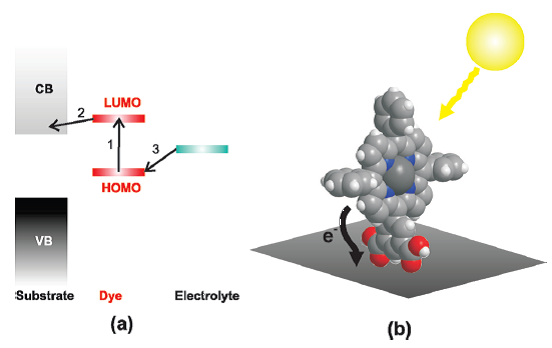 Dye sensitized solar cells (DSSCs) have
been shown to convert solar light into electricity with promising
efficiencies and have attracted considerable interest in the
fundamental aspect of their operation. At the heart of the device is
a thin oxide film, composed of a network of nanocrystalline
TiO2 particles, deposited on a transparent conducting
substrate and sintered so as to establish an effective conduction
path. This nanoporous structure is then sensitized with an organic
dye molecule and infiltrated with an electrolyte, which, in turn,
makes contact to a counter electrode. As the band gap of
TiO2 is over 3 eV, only ultraviolet radiation directly
produces electron-hole pairs in the native material. However, with
the appropriate alignment of the electronic levels, sensitization by
a chemisorbed dye molecule capable of harvesting photons of energy
smaller than the TiO2 band gap, enables efficient
absorption across a large fraction of the solar spectrum. Figure (a)
schematically represents the ground-state energy alignment between
the three main components of DSSCs: a wide band gap semiconductor
substrate, a dye molecule chemisorbed onto this substrate, and an
electrolyte in contact with the dye. Focusing on the dye/oxide
interface, a light-harvesting dye molecule can absorb a photon from
within the visible region of the solar spectrum, resulting in a
photoexcitation that can, in the simplest terms, be thought of as
the elevation of an electron from the highest occupied molecular
orbital (HOMO) to the lowest unoccupied molecular orbital (LUMO) of
the dye (Figure (a), step 1). If the LUMO is degenerate with the
substrate conduction band, the excited electron can transfer to the
substrate (Figure (a), step 2) and participate in current flow across
the cell. The resulting singly occupied HOMO of the oxidized dye can
then be filled via an appropriately chosen electrolyte (Figure (a), step 3). From this,
it is clear that the performance of DSSCs depends strongly on the
relative alignment of the dye molecular levels with respect to the
substrate band edges. Using XPS, UPS and IPS, we can determine the
HOMO-LUMO positions relative to the substrate band structure. The
geometry of the dye molecule at the surface is also studied using
scanning tunneling microscopy and NEXAFS spectroscopy.
Dye sensitized solar cells (DSSCs) have
been shown to convert solar light into electricity with promising
efficiencies and have attracted considerable interest in the
fundamental aspect of their operation. At the heart of the device is
a thin oxide film, composed of a network of nanocrystalline
TiO2 particles, deposited on a transparent conducting
substrate and sintered so as to establish an effective conduction
path. This nanoporous structure is then sensitized with an organic
dye molecule and infiltrated with an electrolyte, which, in turn,
makes contact to a counter electrode. As the band gap of
TiO2 is over 3 eV, only ultraviolet radiation directly
produces electron-hole pairs in the native material. However, with
the appropriate alignment of the electronic levels, sensitization by
a chemisorbed dye molecule capable of harvesting photons of energy
smaller than the TiO2 band gap, enables efficient
absorption across a large fraction of the solar spectrum. Figure (a)
schematically represents the ground-state energy alignment between
the three main components of DSSCs: a wide band gap semiconductor
substrate, a dye molecule chemisorbed onto this substrate, and an
electrolyte in contact with the dye. Focusing on the dye/oxide
interface, a light-harvesting dye molecule can absorb a photon from
within the visible region of the solar spectrum, resulting in a
photoexcitation that can, in the simplest terms, be thought of as
the elevation of an electron from the highest occupied molecular
orbital (HOMO) to the lowest unoccupied molecular orbital (LUMO) of
the dye (Figure (a), step 1). If the LUMO is degenerate with the
substrate conduction band, the excited electron can transfer to the
substrate (Figure (a), step 2) and participate in current flow across
the cell. The resulting singly occupied HOMO of the oxidized dye can
then be filled via an appropriately chosen electrolyte (Figure (a), step 3). From this,
it is clear that the performance of DSSCs depends strongly on the
relative alignment of the dye molecular levels with respect to the
substrate band edges. Using XPS, UPS and IPS, we can determine the
HOMO-LUMO positions relative to the substrate band structure. The
geometry of the dye molecule at the surface is also studied using
scanning tunneling microscopy and NEXAFS spectroscopy.
|
Recent Highlight:
Energy Alignment of a ZnTPP-Ipa on the TiO2(110) and
ZnO(11-20) surfaces
 Metalloporphyrins play an essential role
in the photosynthetic process and therefore, are attractive
candidates for photoinduced electron-transfer mediators in DSSCs.
Among metalloporphyrins, zinc tetraphenylporphyrin (ZnTPP)
derivatives have been found to have similar electron injection and
charge recombination properties as N3 dye, the standard
ruthenium-containing dye used for DSSCs while exhibiting reasonable
performances using either nanostructured TiO2 or ZnO as
substrates. Nevertheless, many fundamental properties of the
dye/metal oxide interface, that is essential for DSSC operation,
such as the electronic structure of the adsorbed dyes, adsorption
geometry and energy level alignment are not well studied and need
careful consideration.
Metalloporphyrins play an essential role
in the photosynthetic process and therefore, are attractive
candidates for photoinduced electron-transfer mediators in DSSCs.
Among metalloporphyrins, zinc tetraphenylporphyrin (ZnTPP)
derivatives have been found to have similar electron injection and
charge recombination properties as N3 dye, the standard
ruthenium-containing dye used for DSSCs while exhibiting reasonable
performances using either nanostructured TiO2 or ZnO as
substrates. Nevertheless, many fundamental properties of the
dye/metal oxide interface, that is essential for DSSC operation,
such as the electronic structure of the adsorbed dyes, adsorption
geometry and energy level alignment are not well studied and need
careful consideration.
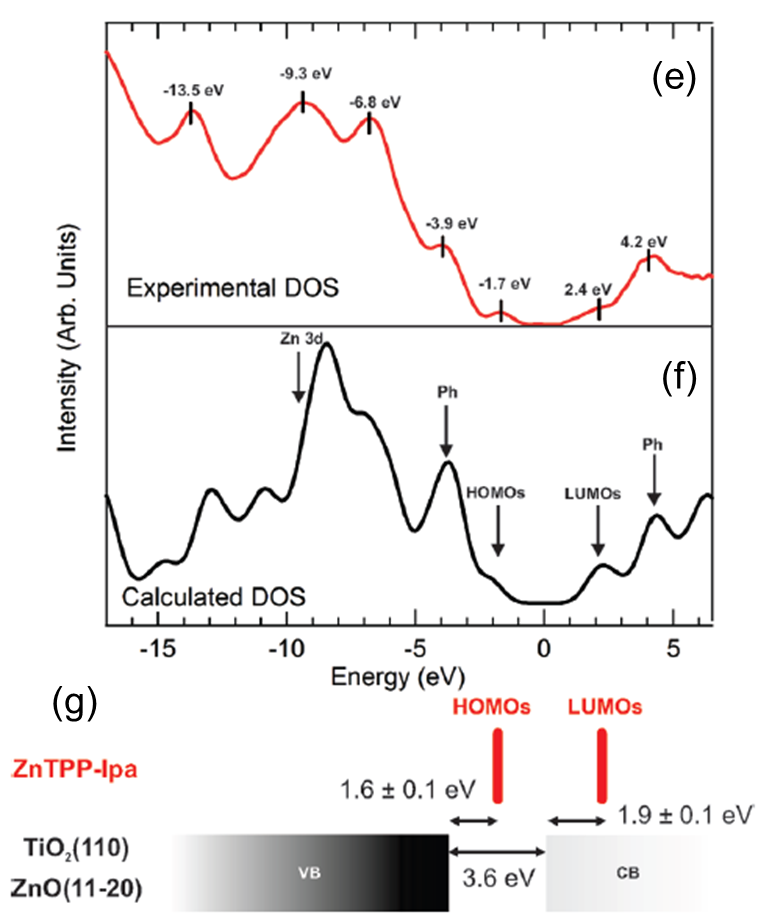 Although in functional dye sensitized solar cells
nanostructured metal oxide thin films are used as a substrate, to
study electronic and geometric properties of the dye/oxide system in
a controlled way, single crystal are used here. The occupied and unoccpied states of both the
oxides surfaces, before and after sensitization with the ZnTPP-Ipa dye (shown in (b))
have been evaluated by means of
UPS and IPS in a single UHV chamber. The measured spectra are shown in
the Figure (c) and (d) for the ZnO(11-20) and TiO2 (110) surfaces.
A comparison of the experimental molecular contribution to the electronic structure (e) with the calculated density
of states (f) of the ZnTPP-Ipa dye, enable the direct determination of the alignment of the moleculat levels with
respect to the substrates band egdes. An energy diagram representative of the dye/oxides interface (g) can be built.
It is found that the HOMOs are located 1.6 eV above the valence band edge and that the LUMOs are situated 1.9 eV above
the conduction band edge of the oxides.
Although in functional dye sensitized solar cells
nanostructured metal oxide thin films are used as a substrate, to
study electronic and geometric properties of the dye/oxide system in
a controlled way, single crystal are used here. The occupied and unoccpied states of both the
oxides surfaces, before and after sensitization with the ZnTPP-Ipa dye (shown in (b))
have been evaluated by means of
UPS and IPS in a single UHV chamber. The measured spectra are shown in
the Figure (c) and (d) for the ZnO(11-20) and TiO2 (110) surfaces.
A comparison of the experimental molecular contribution to the electronic structure (e) with the calculated density
of states (f) of the ZnTPP-Ipa dye, enable the direct determination of the alignment of the moleculat levels with
respect to the substrates band egdes. An energy diagram representative of the dye/oxides interface (g) can be built.
It is found that the HOMOs are located 1.6 eV above the valence band edge and that the LUMOs are situated 1.9 eV above
the conduction band edge of the oxides.
|
Recent Publications
(back to top)
-
A sensitized Nb2O5 photoanode for hydrogen production in a dye-sensitized photoelectrosynthesis cell
H. Luo, W. Song, P.G. Hoertz, K. Hanson, R. Ghosh, S. Rangan, M.K. Brennaman, J.J. Concepcion, R.A. Binstead, R.A. Bartynski, R. Lopez, and T.J. Meyer, Chemistry of Materials 25, 122 (2013)
-
Energy alignment, molecular packing, and electronic pathways: Zinc(II) tetraphenylporphyrin derivatives adsorbed on TiO2(110) and ZnO(11-20) surfaces
S. Rangan, S. Coh, R.A. Bartynski, K.P. Chitre, E. Galoppini, C. Jaye, and D. Fischer, Journal of Physical Chemistry C 116, 23921 (2012)
-
Increasing photocurrents in dye sensitized solar cells with tantalum-doped titanium oxide photoanodes obtained by laser ablation
R. Ghosh, Y. Hara, L. Alibabaei, K. Hanson, S. Rangan, R.A. Bartynski, T.J. Meyer, and R. Lopez, ACS Applied Materials and Interfaces 4, 4566 (2012)
-
Energy alignment of catechol adsorbed on TiO2(110) and ZnO(11-20) surfaces
Sylvie Rangan, Jean-Patrick Theisen and Eric Bersch, Robert Allen Bartynski,
Applied Surface Science,
256,
4829
(2010)
-
Energy level alignment of a Zinc(II)Tetraphenylporphyrin dye adsorbed onto TiO2(110) and ZnO(11-20) surfaces
Sylvie Rangan, Senia Katalinic, Ryan Thorpe, Robert Allen Bartynski, Jonathan Rochford and Elena Galoppini,
J. Phys. Chem. C,
114,
1139
(2010)
|
|

