|

DEPARTMENT OF PHYSICS AND
ASTRONOMY
LINKS
|

Basics: Nanofaceted Metal Surfaces as Model Nanocatalysts and Nanotemplates
In our group, surface faceting is used to prepare a nanoscale model catalyst for surface reactions and a nanoscale template for growth of metallic nanoclusters. Faceting, a form of nanoscale self-replication, occurs on atomically-rough single-crystal metal surface upon annealing adsorbate-covered surface or annealing the surface in the presence of adsorbate (e.g. gas) so that initially planar metal surface converts to a "hill and valley" structure with pyramidal or ridge-like features exposing new crystal faces of nanometer scale dimensions. Faceting is driven by minimization of surface free energy but limited by kinetics of surface diffusion.
|
Recent Highlights: Growth of gold nanoparticles on faceted O/Ru(11-20)
|
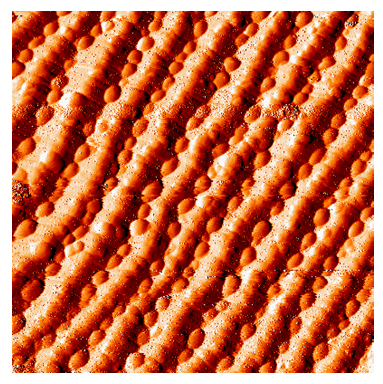
Faceted O/Ru(11-20) is used as a nanotemplate for the growth of gold
nanoparticles. The nanotemplate consists of parallel and uniform ridges
with dimensions ~6nm wide and >200nm long. By depositing Au onto the
nanotemplate surface held at room temperature, Au nanoparticles with
regular spacings and uniform size are fabricated on this nanotemplate. Au
nanoparticles nucleate preferentially within valleys of the
faceted surface and their size can be controlled by changing Au coverage.
This finding provides new insights into synthesis of practical gold
catalysts with high reactivity and selectivity.
|
|
Recent Highlights: Reduction of NO by CO on planar and faceted Ir(210)
Catalytic removal of NO has received considerable attention because of its key role in reducing air pollution from automobile exhaust gases. Of practical importance is reduction of NO by CO since CO is also present in the exhaust gases. Pt-, Pd-, and Rh-based catalysts have been investigated the most for the reduction of NO by CO due to their applications in current commercial three-way catalysts. Recently, it has been shown that Ir/ZSM-5 is much more active than Pt/ZSM-5 for the reduction of NO by CO in the presence of excess oxygen, which signifies a potential application of Ir as a new exhaust catalyst for current diesel and lean-burn engines.
We have investigated reduction of NO by CO on clean planar Ir(210) and clean faceted Ir(210) with tunable facet sizes (5-14nm), prepared from the same crystal in situ, with a focus on structural and size effects in the reaction. Nanoscale three-sided pyramids exposing (311), (31-1) and (110) faces on each pyramid are formed on an initially planar Ir(210) surface upon annealing in oxygen at T=>600K, and a clean faceted Ir(210) surface can routinely be prepared in situ by reaction with hydrogen at 400K.
We have used temperature programmed desorption (TPD) to monitor the reaction products. Both planar and faceted Ir(210) are highly active for reduction of NO by CO with high selectivity to N2, which is accompanied by simultaneous oxidation of CO to CO2. Evidence is found for structure sensitivity in the reactions on faceted Ir(210) versus planar Ir(210). Strong interaction between NO and CO at high NO exposure and 1ML CO pre-coverage results in "explosive" evolution of N2 and CO2 on planar Ir(210) and size effects in reduction of NO by CO on faceted Ir(210) for average facet size ranging from 5 to 14nm without change in facet structure.
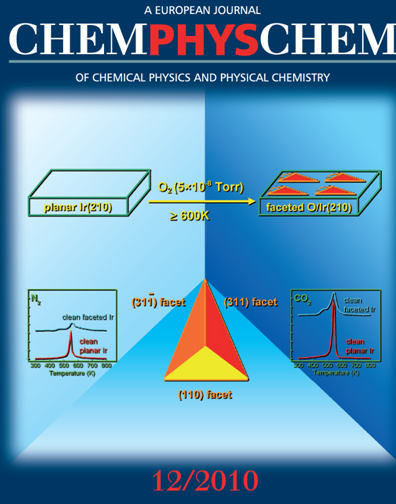
In the cover picture, the top figure shows a schematic of the process to prepare a faceted O/Ir(210) surface. The middle bottom figure shows a schematic of a single pyramid from the faceted Ir(210) surface. The figures on the sides of the bottom show TPD spectra of N2 and CO2 from adsorption of NO on clean planar Ir(210) and clean faceted Ir(210) pre-exposed to CO.
Read more:
Reduction of NO by CO on unsupported Ir: Bridging the materials gap,
Wenhua Chen, Quantong Shen, Robert A. Bartynski, Payam Kaghazchi, and Timo Jacob, ChemPhysChem,
11, 2525 (2010)
|
Recent Publications
(back to top)
-
Nano-faceted metal surfaces: Structure, reactivity and applications (invited review paper)
Wenhua Chen, Hao Wang,
and Robert A. Bartynski, in book: Catalysis by Materials with Well-
defined Structures, Elsevier B.V., the Netherlands,
(ISBN: 978-0-12-801217-8), Editors: Z. Wu and S. H. Overbury, pp.301-338, 2015.
-
Oxygen-induced nano-faceting of Re(11-21)
Hao Wang, Wenhua Chen,
and Robert A. Bartynski, Surface Science, 635, 85 (2015)
-
Selective oxidation of ammonia by co-adsorbed oxygen on iridium surfaces: Formation of N2O
Wenhua Chen, Quantong Shen,
and Robert A. Bartynski, Catalysis Letters, 145, 757 (2015)
-
Theoretical study of carbon adsorption on Re surfaces: Morphological instability
Payam Kaghazchi, Timo Jacob, Xiaofang Yang, Grant Junno, Hao Wang, Wenhua Chen, Bruce E. Koel, and Robert
A. Bartynski, Catalysis Letters,
144, 1667 (2014)
-
Nitrogen-induced reconstruction and faceting of Re(11-21)
Hao Wang, Wenhua Chen, Robert A. Bartynski, Payam Kaghazchi, and Timo Jacob
, Journal of Chemical Physics,
140, 024707 (2014)
-
Morphological stability of oxygen- and nitrogen-covered Ru(11-21)
Quantong Shen, Wenhua Chen, Hao Wang, and Robert. A. Bartynski, Journal of Chemical Physics,
139, 084707 (2013)
-
Theoretical and experimental studies of hydrogen adsorption and desorption on Ir surfaces
Payam Kaghazchi, Timo Jacob, Wenhua Chen, and Robert. A. Bartynski, Physical Chemistry Chemical Physics,
15, 12815 (2013)
-
Reduction of nitric oxide by acetylene on Ir surfaces with different morphologies:
comparison with reduction of NO by CO
Wenhua Chen, Quantong Shen, Robert A. Bartyns
ki, Payam Kaghazchi, and Timo Jacob,
Langmuir, 29, 1113 (2013)
-
Oxidation of CO by NO on planar and faceted Ir(210)
Wenhua Chen, Robert A. Bartynski, Payam Kaghazchi, and Timo Jacob,
Journal of Chemical Physics, 136, 224701 (2012)
-
Nano-faceted C/Re(11-21): fabrication, structure and template for synthesizing nanostructured model Pt electrocatalyst for hydrogen evolution reaction
Xiaofang Yang, Bruce E. Koel, Hao Wang, Wenhua Chen, and Robert A. Bartynski,
ACS Nano, 6, 1404 (2012)
-
Growth of gold nanoparticles on faceted O/Ru(11-20) nanotemplate
Quantong Shen, Wenhua Chen, and Robert A. Bartynski,
Surface Science, 605, 1457 (2011)
-
Reduction of NO by CO on unsupported Ir: Bridging the materials gap
Wenhua Chen, Quantong Shen, Robert.A. Bartynski, P. Kaghazchi, and T. Jacob,
ChemPhysChem, 11, 2515 (2010) (Featured on the front cover of the issue 12/2010 and highlighted in the news section of ChemPhysChem)
-
Nano-faceting of the Ru(11-20) surface
Quantong Shen, Wenhua Chen, Hao Wang, Govind, Theodore E. Madey, and Robert A. Bartynski, Surface Science, 604, L12 (2010)
-
Adsorption and decomposition of NO on O-covered planar and faceted Ir(210)
Wenhua Chen, Alan L. Stottlemyer, Jingguang G. Chen, Payam Kaghazchi, Timo Jacob, Theodore E. Madey, and Robert A. Bartynski, Surface Science, 603, 3136 (2009)
|
|



